Microflow LC-MS for Clinical Proteomics & Metabolomics
Robust, reproducible workflows for clinical research that overcome immunoassay limitations.
LC-MS as a Core Technology for Clinical Analysis
Liquid chromatography–mass spectrometry (LC-MS) has become an essential analytical technology in clinical research and translational studies. While immunoassays such as ELISA remain widely used in clinical diagnostics, LC-MS provides molecular specificity that is difficult to achieve with antibody-based methods, particularly for small molecules and proteins with post-translational modifications (PTMs).
ELISA vs LC-MS: Complementary Capabilities in Clinical Research
| Capability | ELISA | LC-MS |
| Small-molecule detection | Generally not feasible | Direct molecular detection |
| Protein isoforms | Limited specificity | Isoform-resolved |
| Post-translational modifications (PTMs) | Often indistinguishable | Directly measureable |
| Multiplexing | Single or limited analytes | Multiple analytes per run |
| Assay development | Antibody-dependent | Method-driven |
Microflow LC-MS: Balancing Sensitivity and Robustness for Clinical Workflows
Microflow LC-MS has emerged as a practical compromise between nanoLC and analytical-flow LC, balancing robustness, reproducibility, and sensitivity for clinical workflows that demand consistent performance over long analytical sequences. It is particularly powerful in analyzing limited amounts of biofluid samples such as blood, plasma, CSF, and urine, as well as tissue biopsies and dry blood spots.
Microflow LC-MS is therefore emerging as a preferred approach for clinical proteomics, metabolomics, and translational research applications.
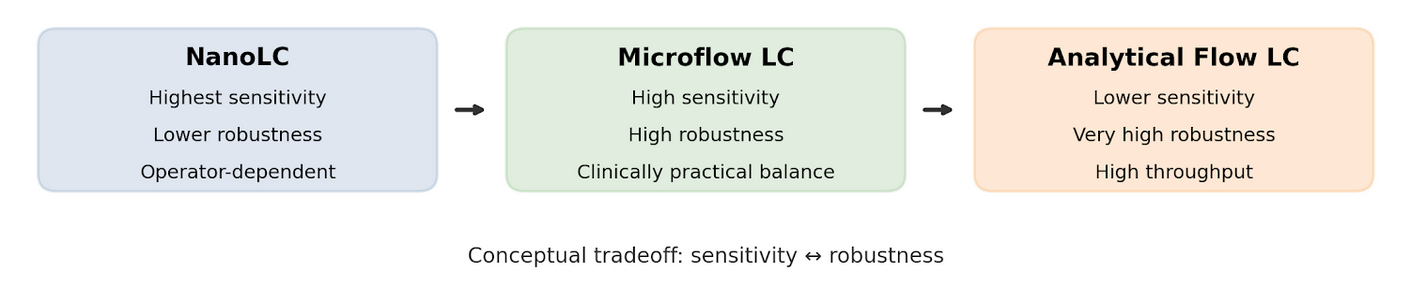
Comparison of LC Flow Regimes and Clinical Suitability
| Parameter | NanoLC | Microflow LC | Analyical Flow LC |
| Typical flow rate | < 1 µL/min | 1-50 µL/min | > 100 µL/min |
| Sensitivity | Very high | High | Moderate |
| Robustness | Low | High | Very High |
| Operator dependence | High | Moderate-low | Low |
| Clinical workflow suitability | Limited by robustness | Strong | Limited by sensitivity |
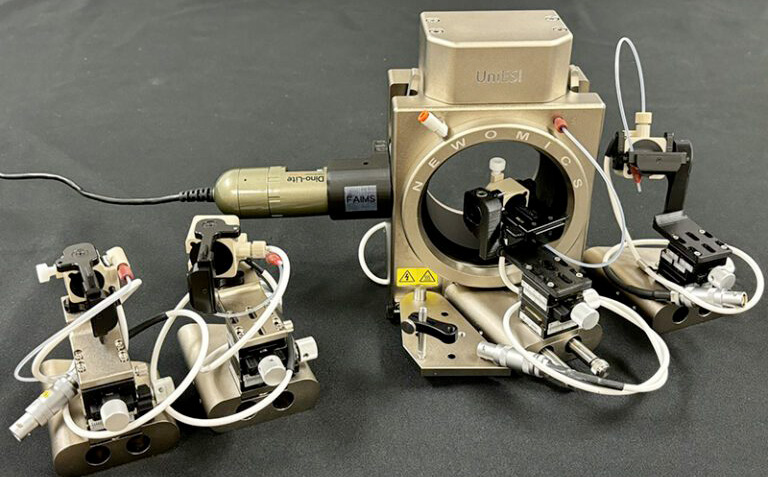
How Newomics Enables Robust Clinical MS Workflows
As clinical laboratories increasingly adopt microflow LC-MS, the LC-MS front-end becomes a critical determinant of sensitivity, robustness, and reproducibility. The Newomics UniESI platform integrates:
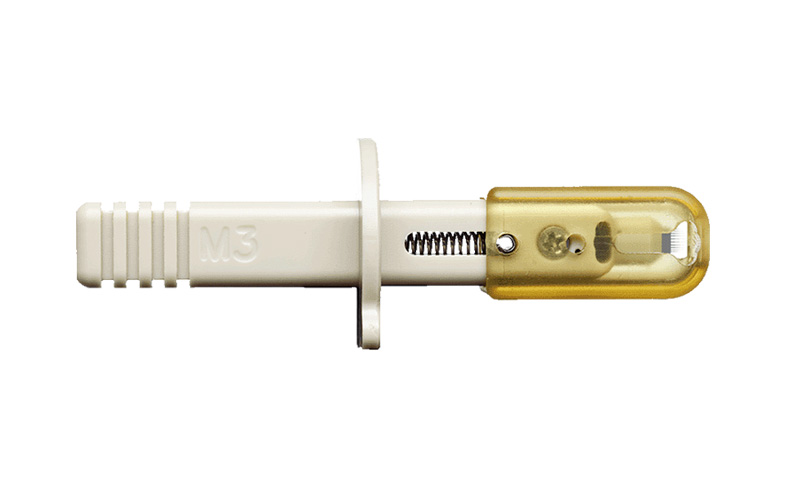
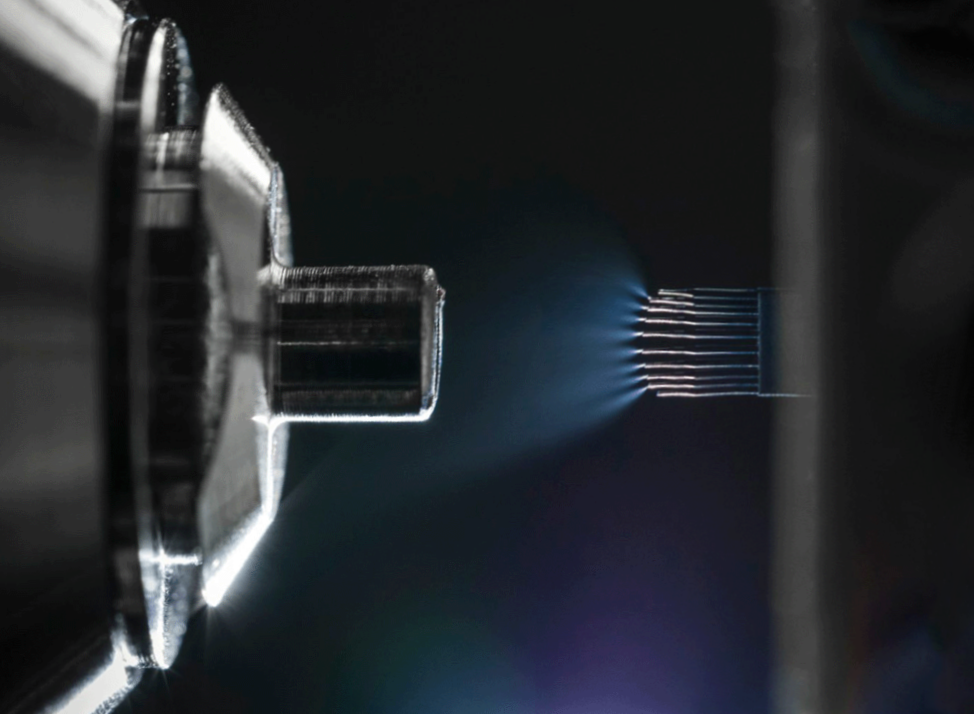
- Patented multinozzle M3 emitters
- MEA microfluidic chips
- Stabilized electrospray performance at microflow rates
- Nanoflow-like sensitivity with microflow robustness
This combination improves tolerance to sample variability and supports the long analytical sequences typical in clinical proteomics and metabolomics.
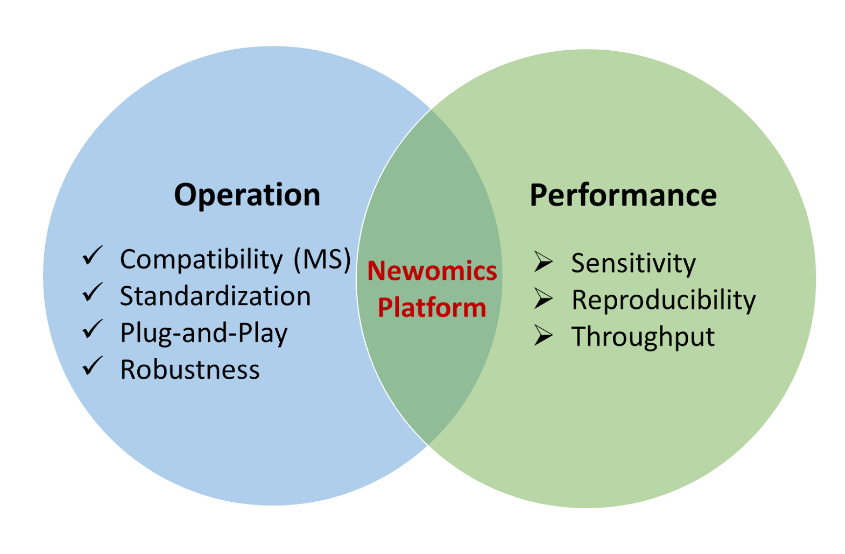
UniESI is vendor-agnostic and compatible with mass spectrometers from five major manufacturers, enabling standardized workflows across heterogeneous clinical instrument fleets.
Newomics Products Enabling Robust Clinical MS
Representative Customer Publications
Selected peer-reviewed publications illustrating the adoption of Newomics UniESI platform for microflow LC-MS in clinical proteomics and metabolomics.
Application Notes and Recent Webinars
Application notes describe validated microflow LC-MS workflows for clinical research, including analysis of proteins with post-translational modifications, targeted proteomics, and quantitative metabolomics.
Recorded webinars and technical discussions provide additional context and practical guidance for implementing microflow LC-MS in clinical MS workflows.
Discuss Your Clinical MS Workflow with Our ScientistsReferences
Selected peer-reviewed publications illustrating the adoption of LC-MS and microflow LC-MS in clinical proteomics and metabolomics:
- On the potential of micro-flow LC-MS/MS in proteomics
- Hyphenation of microflow chromatography with electrospray ionization mass spectrometry for bioanalytical applications focusing on low molecular weight compounds: A tutorial review
- Multiplex Mass Spectrometry Analysis of Amyloid Proteins in Human Plasma for Alzheimer’s Disease Diagnosis
- Plasma Proteome Profiling to Assess Human Health and Disease
- Standardized Workflow for Precise Mid- and High-Throughput Proteomics of Blood Biofluids
- Recent developments in mass-spectrometry-based targeted proteomics of clinical cancer biomarkers
- A Comprehensive LC–MS Metabolomics Assay for Quantitative Analysis of Serum and Plasma
- Standard operating procedure combined with comprehensive quality control system for multiple LC-MS platforms urinary proteomics
- Evolution of LC–MS/MS in clinical laboratories
Note: Content intended for clinical research and translational applications.