Native MS Kit
The Native MS kit is a ready-to-use solution to start analyzing proteins in their native state. The kit is made to study Native MS with different approaches:
- With Column –> to identify proteins and protein complexes in their native state with
- With direct Infusion –> to analyze the proteins and protein complexes in their native state with direct infusion ESI
In combination with the M3 Emitter and MnESI ion source, the Native MS Kit provides exceptional sensitivity, robustness, and throughput, making it an ideal microflow LC-MS platform for native MS analysis.
The Native MS kit is a ready-to-use solution to start analyzing proteins in their native state. The kit is made to study Native MS with different approaches:
- With Column –> to identify proteins and protein complexes in their native state with
- With direct Infusion –> to analyze the proteins and protein complexes in their native state with direct infusion ESI
In combination with the M3 Emitter and MnESI ion source, the Native MS Kit provides exceptional sensitivity, robustness, and throughput, making it an ideal microflow LC-MS platform for native MS analysis.
Product Details
Model: NAK-01
Features & Benefits
- 1-20 µL per min flow rate
- Better ionization: more charge available for each smaller droplet (via multinozzle) during electrospray ionization process
- Lower voltage compared to high flow: softer ionization to maintain the protein, protein-drug interactions, and protein complexes in the native states
- Room temperature for desolvation gas: maintain the protein, protein-drug interactions, and protein complexes in the native states.
Specifications
| Parameter | Specifications |
|---|---|
| Product | Native MS Kit for Microflow LC/MS, 1 – 20 µL/min |
| Catalog # | NAK-01 |
| Column set includes | PolyHydroxyethylA capillary, 150x0.30 mm, 3μm, 300-Å; PolyHydroxyethyla 150x0.30 mm, 3μm, 1000-Å |
| Direct Infusion set includes | 500 µl Gastight Syringe; 100 µl Gastight Syringe |
| Warranty | 3 months |
Performance Highlight
Read Application Note
The Newomics MnESI platform was applied for intact NIST- mAb characterization under native conditions. Nebulizer gas flow, dry gas flow, dry temperature, isCID, and other MS acquisition parameters were optimized for the native NISTmAb characterization.
As illustrated by the extracted ion chromatogram (m/z 5000 – 6500) in Figure A, the NISTmAb was eluted at 4.1 min.
The mass spectra in Figure B shows that a native charge distribution envelope was observed with the corresponding most abundant charge state of 26+ suggesting the native conformation of NISTmAb was well preserved with the Newomics MnESI. The deconvoluted mass spectrum of NISTmAb (Figure C) shows all major glycoforms of NISTmAb were identified and this further demonstrated enhanced desolvation and adduct removal can be achieved with the Newomics MnESI source.
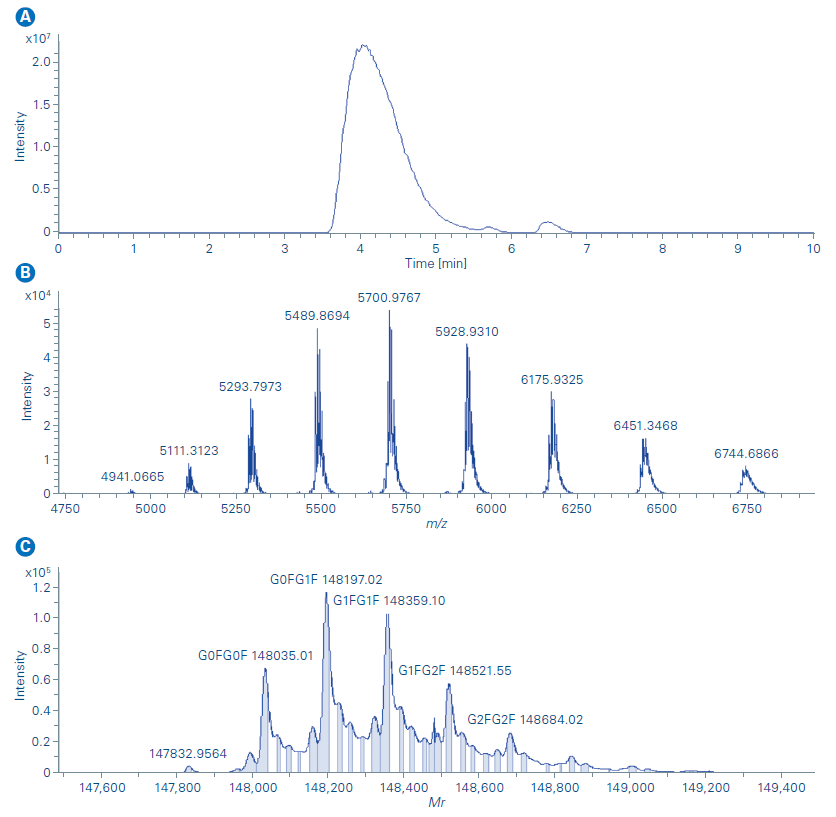
A. Extracted ion chromatogram (m/z 5000 – 6500) for capillary flow SEC of NISTmAb at 500 µg/mL.
B. Mass spectrum of NISTmAb.
C. Deconvoluted mass spectrum of NISTmAb.
Read Application Note
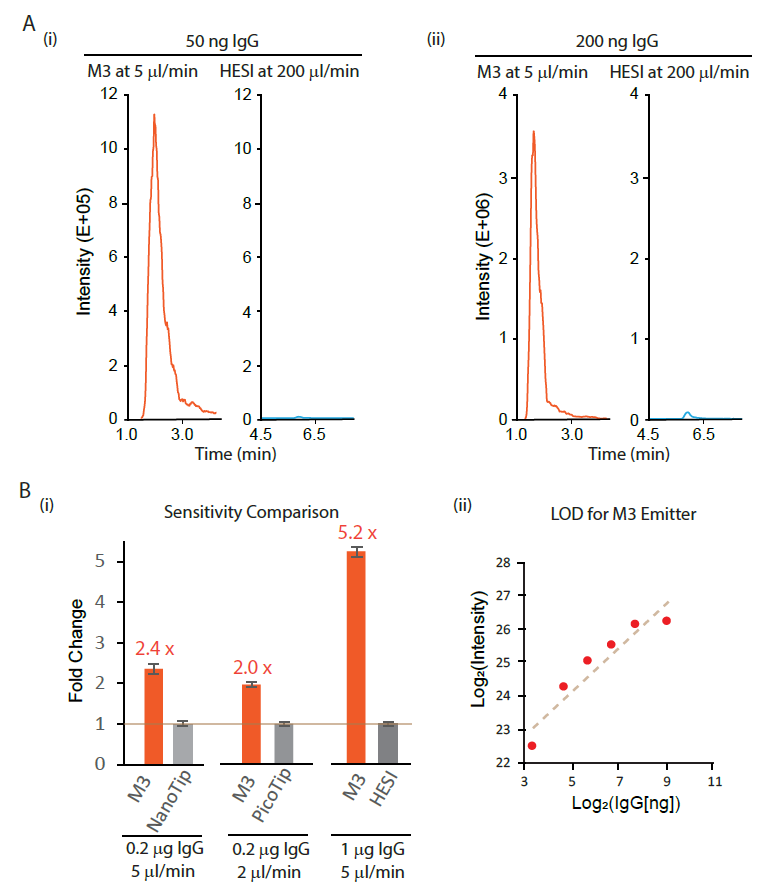
The M3 Emitter with microflow SEC-MS was demonstrated to gain more than a 10-fold sensitivity gain over high-flow SEC-MS by HESI using various amounts of IgG (Fig. Ai). Additionally, the IgG retention period from microflow SEC with the M3 emitter was substantially shorter than that from high-flow SEC with the HESI emitter (Fig. Aii).
Then, we compared M3 emitters with NanoTip, PicoTip, and HESI, coupled with the same SEC column. For the comparison to PicoTip, we chose a 2 µl/min flow rate due to the high back pressure from the single nozzle 10 mm ID PicoTip for higher flow rates. On average, we observed a 2.4-fold sensitivity gain of M3 emitters over NanoTip at 5 µl/min, a 2-fold sensitivity gain over PicoTip at 2 µl/min, and a 5.2-fold sensitivity gain over HESI at 5 µl/min (Fig. Bi).
Finally, we measured the limit of detection (LOD) for microflow SEC-MS analysis of native IgG using M3 emitters. A dilution curve analysis shows excellent linearity of MS signals on the mAb amount from 25 to 200 ng, with a LOD value below 10 ng IgG (Fig. Bii)
Read Application Note
GroEL is a molecular chaperone with a molecular weight of around 801 kDa (14-mer, 57 kDa each). It’s a fully active tetradecamer complex that’s needed for the appropriate folding of numerous proteins in cells, as well as a common model protein for tuning and optimizing mass spectrometers for native MS analysis. We performed the 3-way comparison, microflow LC-MS after 1:10 splitting from analytical flow, analytical flow LC-MS, and static NanoESI-MS.
As illustrated by the total ion chromatogram (TIC) of the GroEL 14-mer (Fig. 3 a), GroEL was eluted as a single peak with a 14-mer peak and its monomer peak was not observed. A similar charge envelope of the native GroEL 14- mer complex was observed using the MnESI source with M3 multinozzle emitters at microflow of 5 µL/min (Fig. 3 b), compared to the charge state envelopes using the HESI source with single nozzle emitters at an analytical flow of 50 µL/min (Fig. 3 c), as well as using the Nanospray Flex source with single nozzle emitters at static nanoflow of <40 nL/min (Fig. 3 d).
Mass spectra from microflow LC/MS analysis using MnESI, analytical flow LC/MS using HESI, and static NanoESI-MS all contained well-resolved, native charge states, and the measured mass corresponded well with the expected mass for the GroEL 14-mer complex of 801 kDa. As shown in Figure 3b, we were able to detect the minor 15-mer GroEL complex (< 5%) as our preparation produced the active form of GroEL.
When compared to the HESI analytical flow approach, the MnESI platform at microflow produced higher signal intensity and lower chemical noise. The signal intensity of the most abundant peak achieved by the MnESI platform was 4.8 times greater than the HESI method, and the signal-to-noise ratio (S/N) for the most abundant peak by MnESI was nearly 11 times higher than the HESI probe method (Fig. 3 e). It’s worth mentioning that the MnESI-MS platform used a 1:10 post-column flow splitting at microflow, which implies the sample volume injected for MS detection was 10 times lower than the sample amount used in the HESI probe analytical flow approach. Considering the amount of protein sample injected into the MS, the MnESI platform demonstrated a significant gain of approximately 50-fold in sensitivity compared to the conventional HESI probe analytical flow method. With the sensitivity improvement from the MnESI platform, it is possible to analyze samples with a smaller amount or shorter gradient. Furthermore, the reduction of high salt and protein complexes injection into the MS minimizes the contamination of the instrument, enhances method robustness, and reduces the downtime due to cleaning.
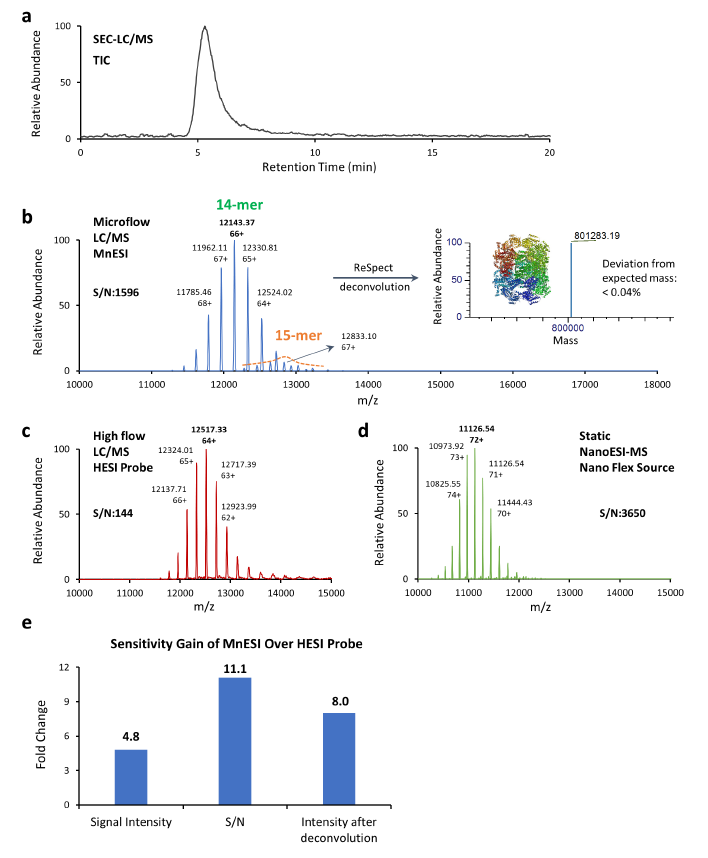
b. Native MS1 analysis of GroEL 14-mer using the MnESI platform at microflow of 5 μL/min. Secondary complex of 15-mer was clearly detected.
c. Native MS1 analysis of GroEL 14-mer using the Ion Max source with HESI probe at analytical flow of 50 μL/min. d. Native MS1 analysis of GroEL 14-mer (1.25 μM) using static NanoESI-MS. e. Sensitivity comparison of MnESI platform vs. HESI probe method.